Nutrient requirements of the yeast cell – Don’t forget about the micronutrients!
Inside a single yeast cell (Saccharomyces cerevisiae) it is estimated that there are over 5000 biochemical reactions involving enzymes. All of these enzymatic reactions have evolved over time in order for the yeast cell to metabolize and multiply. In the fuel ethanol industry however, the production of ethanol from glucose is accomplished with only 13 of these biochemical reactions. Despite this fact, nearly all of the yeast cell enzymes require a mineral cofactor bound to the enzyme in order for the enzyme to work.
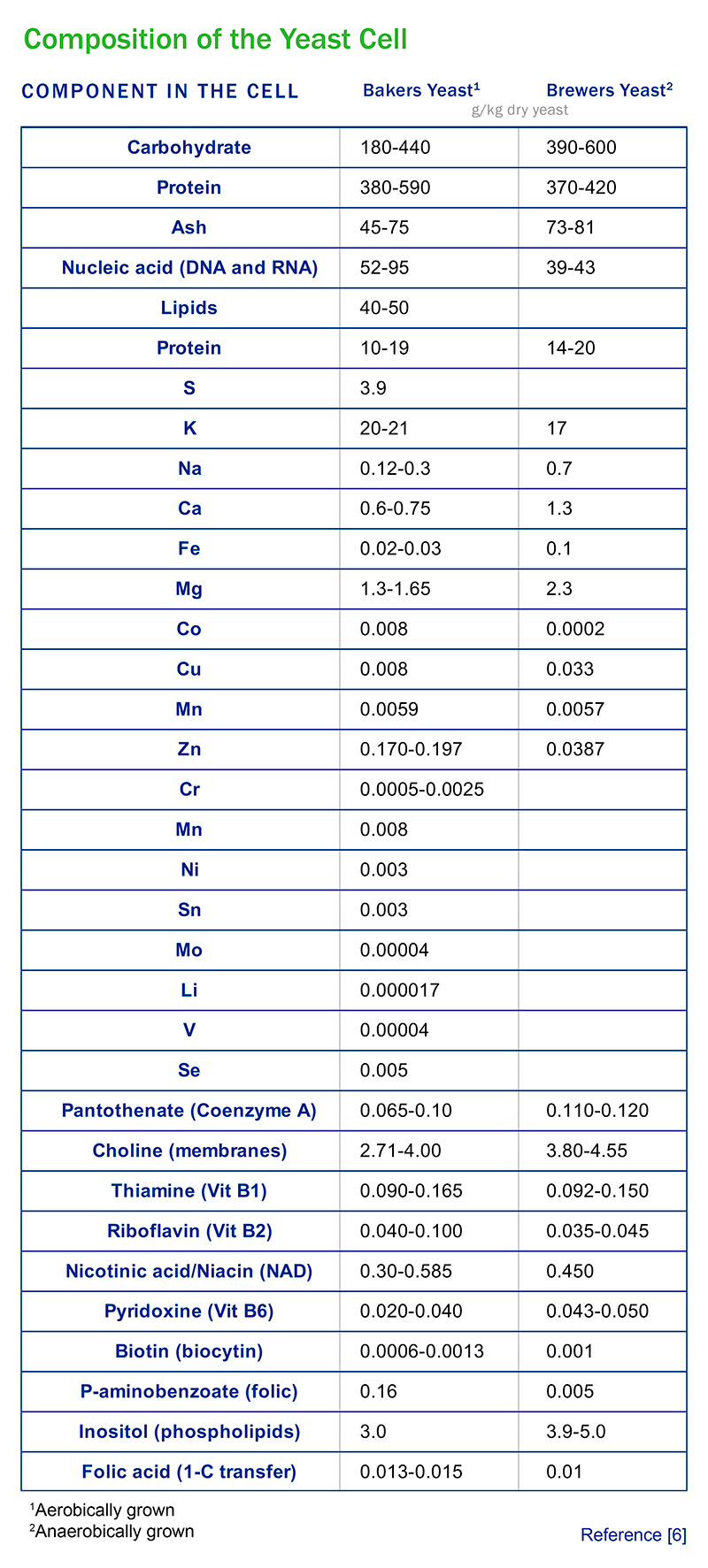 Basic composition of the yeast cell
Basic composition of the yeast cell
Macronutrients are elements or minerals that are required by the yeast cell in relatively large amounts. Carbon, hydrogen, and oxygen (as carbohydrates) collectively comprise ~ 45% grams dry weight (gdw) of a typical yeast cell. Carbohydrates are supplied by sugars in the mash. Nitrogen comprises ~ 9% gdw in a yeast cell. All corn mash is deficient in nitrogen in the form of FAN (free amino nitrogen) – the only form of nitrogen the yeast cell can metabolize. Common FAN chemicals that are typically added in fuel ethanol plants are urea, aqueous ammonia, and amino acids (released by proteases or added as a yeast food). Typically, a minimum FAN level of 600 ppm in the propagator and 300 ppm in the fermentors is required. Biochemically, nitrogen is needed as an element within proteins as a chemical component (all amino acids which are the building blocks of proteins contain at least one nitrogen atom).
Potassium comprises ~ 3.5% gdw in a yeast cell and is needed for many enzymes in the yeast including enzymes on the yeast cell membrane that help produce energy (ATP) for the yeast. Phosphorus comprises ~ 2% gdw in the yeast cell and is critical in yeast cell wall components, energy production (ATP), and components in nucleic acids. Sulfur comprises ~ 0.5% gdw in a yeast cell and is essential both in the composition of methionine and cysteine (two amino acids that make up proteins), and as a cofactor for certain enzymes. All together, C, H, O, N, K, P, and S total ~ 60% of the yeast gdw.
What about the remaining composition of the yeast cell? Micronutrients including other minerals and vitamins make up this remainder. To illustrate this point, proteins (containing C, H, O, N, and S) comprise over 59% of the dry weight of a yeast cell, but the total amount of minerals in a typical yeast cell is less than 4%.
To fully optimize fuel ethanol fermentation by yeast, it seems reasonable to focus mre on macronutrients than micronutrients. However, decades of research into yeast micronutrient requirements clearly shows that micronutrients cannot be ignored. Two critical micronutrients that the yeast requires are zinc and magnesium.
Zinc
Zinc comprises only 0.13% gdw of the composition of the yeast cell but despite this fact, zinc has multiple roles in the yeast cell and is important to an efficient and profitable commercial ethanol fermentation. Three enzymes from the 13 enzymes that convert glucose to ethanol (alcohol dehydrogenase, aldolase, acetaldehyde dehydrogenase) critically require zinc as a cofactor. Corn mash at 32% solids typically contains 6.58 ppm zinc. The yeast nutritional demand for zinc in a 750,000 gal fermentor when inoculated with 300 x106 cells/ml from the propagator calculates to be 0.98 ppm. Thus on its own, corn mash will usually provide the basic nutritional needs of yeast with zinc.
Providing excess zinc (above 6.58 ppm) provides the yeast with additional benefits and capabilities. In studies where zinc was added at concentrations from 14 to 26 ppm, the ethanol yield from yeast increased by 0.1 %w/v (absolute) to 0.32 % w/v (absolute) [1]. Excess zinc not only boosts ethanol production, but can also increase uptake of maltose and maltotriose (from starch hydrolysis). Increased tolerance to acetic acid, ethanol, heat shock, osmotic shock, oxidative stress, and salt stress (halotolerance) have also been documented in yeast provided with excess zinc [4] [8] [9] .For cellulosic ethanol production utilizing dilute acid to hydrolyze fiber (cellulose and hemicellulose), excess zinc has been linked to increased yeast tolerance to hydroxymethylfurfural (HMF), a byproduct with high toxicity..
Magnesium
Magnesium comprises only 0.5% gdw of the composition of the yeast cell, and like zinc, plays critical roles in the yeast cell. More than 300 enzymes within the yeast cell require magnesium in order to function. Corn mash at 32% solids typically contains ~ 658 ppm magnesium. The yeast nutritional demand for magnesium in a 750,000 gal fermentor when inoculated with 300 x106 cells/ml from the propagator calculates to be 97 ppm. Again as with zinc, the corn mash will usually provide the basic nutritional needs of yeast with magnesium.
How about adding excess magnesium? Excess magnesium will also stimulate the production of ethanol from yeast. In studies where magnesium was added to yeast fermentations at 500 ppm, the final ethanol concentration increased by 0.1% w/v absolute [3] [7] . Other published studies confirm this. Excess magnesium not only boosts ethanol yield, but can also regulate yeast cell growth, cell division, and size of the yeast cell. Magnesium also protects yeast cell membranes against stresses caused by high temperatures, ethanol, and osmotic pressure [2] [9] . The fluidity of the yeast cell membrane (critical in preventing toxic chemicals from entering the yeast cell) is regulated in part by magnesium to help prevent “leaking cells”. Stress proteins are produced by the yeast as an internal signal to environmentally stressful conditions (heat, osmotic pressure, etc). Magnesium helps mitigate the production of these signal proteins – “calming the yeast down”. A unique property of excess magnesium is that it can aid the yeast cell to detoxify against heavy metals ions (e.g. Sn2+, Hg2+, Co2+, Cr2+, Pb2+, Cu2+, and Cd2+). In fermentations where these heavy metal ions were present, excess magnesium (to 1000 ppm) decreased the toxic effects of these ions and recovered ethanol production up to 65% of the controls [5] [7] .
Adding yeast foods to fermentation
These two micronutrients (zinc and magnesium) help to illustrate that although adequate nutrition will permit the yeast to grow, supplementing the fermentation with additional micronutrients can empower the yeast to perform better either by accelerating growth, filling in nutritional gaps due to processing specifics, and/or nutrient scavenging by bacterial contamination. Yeast “food” supplements should be added as a preventive measure to overcome any deficiencies and enable consistent and maximum ethanol production by the yeast.
References
- D.P. Bayrock. 2012. Interactions and influences of anionic and cationic chemicals on yeast – positive and negative effects with chemical type and concentrations on yeast nutrition, growth, and fermentation. .
- Rosslyn M. Birch and Graeme M. Walker. 2000. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzyme and Microbial Technology 26, 9–10: 678–687.
- Sylwia Bonin. 2014. Effects of magnesium ions on both VHG batch and continuous fruit wine fermentations: Effects of magnesium ions on fruit wine fermentations. Journal of the Institute of Brewing: 477–485.
- Cheng Cheng, X. Zhao, and F. W. Bai. 2016. Effects of cell flocculation and zinc sulfate addition on acetic acid stress tolerance of Saccharomyces cerevisiae. Chinese Journal of Applied Environmental Biology 22, 1: 0116–0119.
- Graeme M. Walker. 1994. The Roles of Magnesium in Biotechnology. Critical Reviews in Biotechnology 14, 4: 311–354.
- W M Ingledew. 1991. Alcohol production. Progress in Industrial Microbiology 10: 129-177.
- Jianlong Wang and Can Chen. 2006. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnology Advances
- Xin-Qing Zhao and Feng-wu Bai. 2012. Zinc and yeast stress tolerance: Micronutrient plays a big role. Journal of Biotechnology 158, 4: 176–183.
- X.Q. Zhao and F.W. Bai. 2009. Mechanisms of yeast stress tolerance and its manipulation for efficient fuel ethanol production. Journal of Biotechnology 144, 1: 23–30.