
Yeast Health
Yeast health is important because strong, aggressive yeast propagate faster and can help reduce the impact of bacterial infection on fermentation. When yeast are numerous, strong, and well established, they can help fermentation thrive.
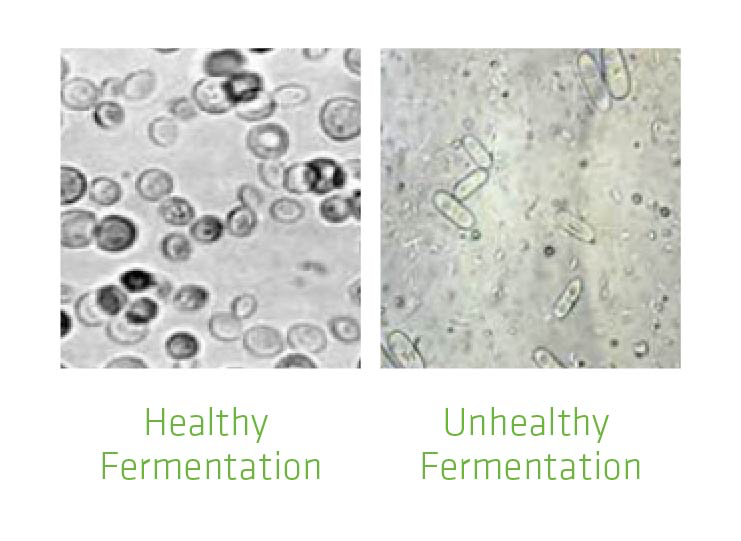
Figure 1 shows yeast cells in healthy vs. unhealthy fermentation. In unhealthy fermentation, the yeast cells are elongated, signaling stress; the smaller organisms are bacteria. If the yeast are weak, bacteria can thrive. In addition, the bacteria also hurt the yeast by producing toxic by-products such as organic acids. Keeping yeast healthy and strong minimizes impact of infection. To understand how to keep yeast healthy, it is helpful to know the basic nutritional requirements of yeast.
Nutrition
For optimal performance, yeast has several nutritional requirements:

Most of these requirements are easily met:
- Water is included in the mash mixture.
- Carbon source comes from the starch in the corn.
- Most plants introduce air into their propagators to boost oxygen.
- Vitamins & Minerals usually come in sufficient amounts from the corn mash.
In contrast, nitrogen is not naturally present in abundance in corn mash. Therefore, plants must find ways to meet the nitrogen needs of yeast in fermentation.
Nitrogen
Yeast cannot consume some forms of nitrogen. It can’t consume elemental nitrogen, or N2, which is in the air. Yeast can only consume nitrogen categorized as Free Amino Nitrogen (FAN). These amino-nitrogen containing chemicals are what yeast need for protein synthesis.
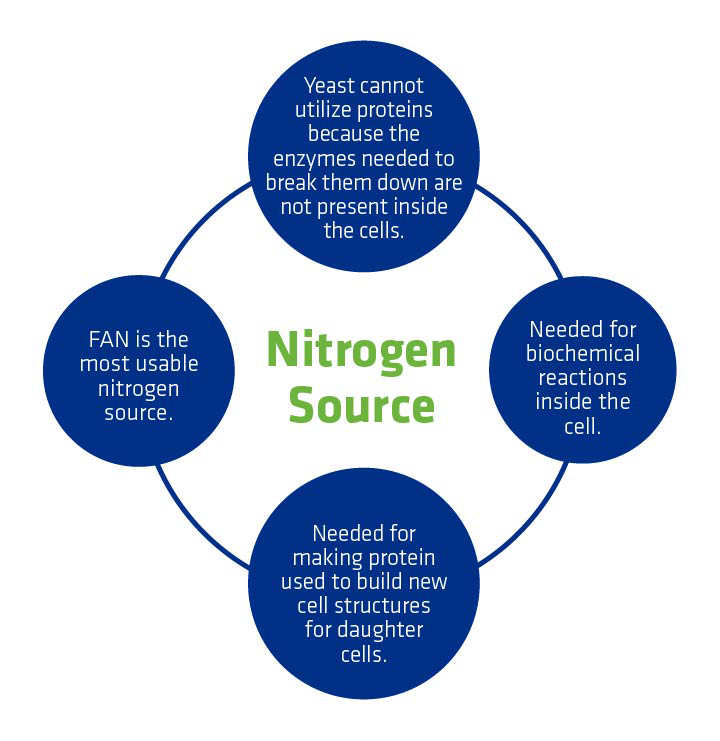
Common nitrogen sources include:
- Ammonia
- Urea
- Amino acids liberated from feedstock proteins by proteases
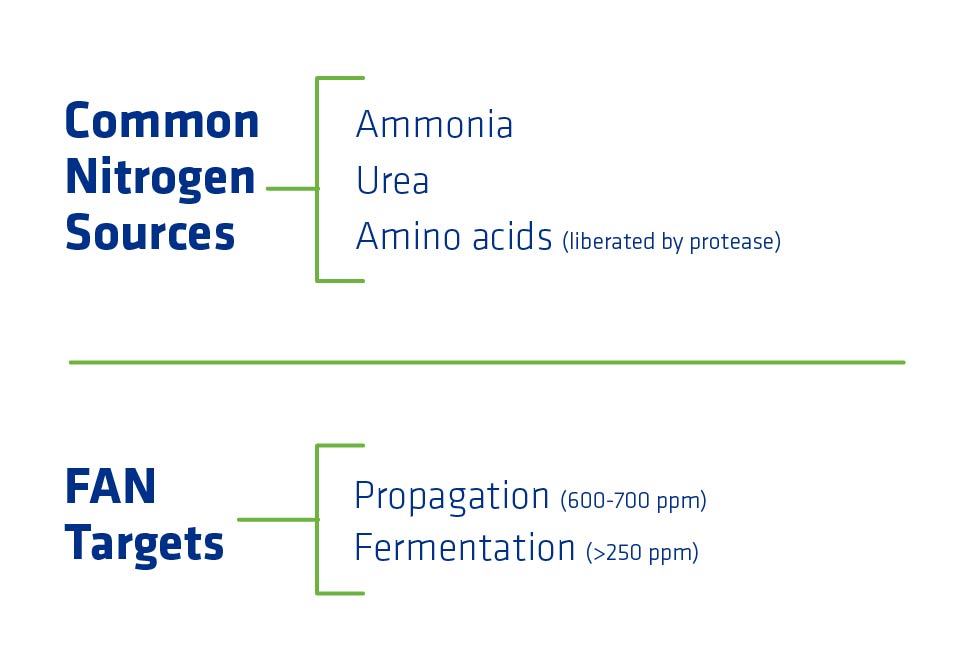
One source of FAN, amino acids, is found in the proteins present in corn. Amino acids are the building blocks of proteins. Yeast cannot uptake or break down corn proteins into amino acids, so ethanol plants must add a type of enzyme called protease to cut up corn proteins into amino acids the yeast can use to make their own proteins. Protease also helps to break the matrix between starches, protein and lipids, so it may help to liberate additional corn oil.
High concentrations of FAN stimulates yeast to work faster. A fairly high concentration of FAN (500 -700 ppm) in propagation will drive the yeast to replicate aggressively, while a more moderate dose of around 250 ppm will be more economical for the much larger fermenter. Plants with shorter fermentation times may benefit from a higher dose of nitrogen.
Identifying Nutrient Deficiency
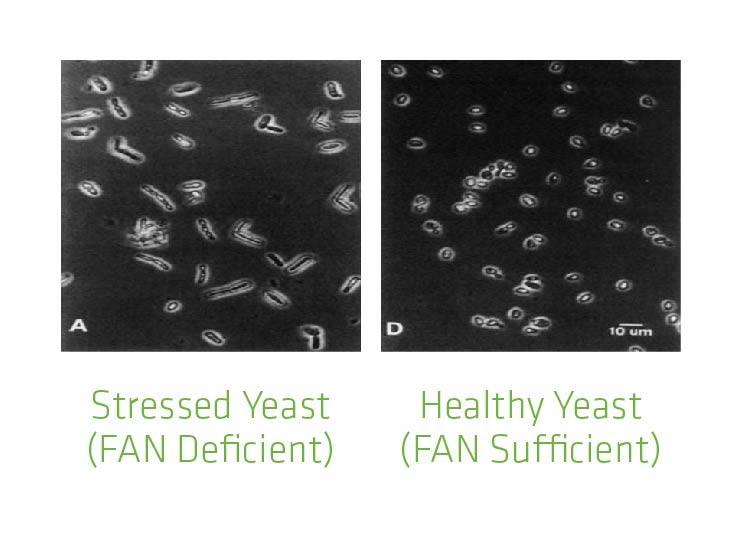
It is possible to identify nutrient deficiency in yeast cells using a microscope. The deficient cells will appear elongated because they are expanding their surface area (effectively stretching) to try to come into contact with the missing nutrients.
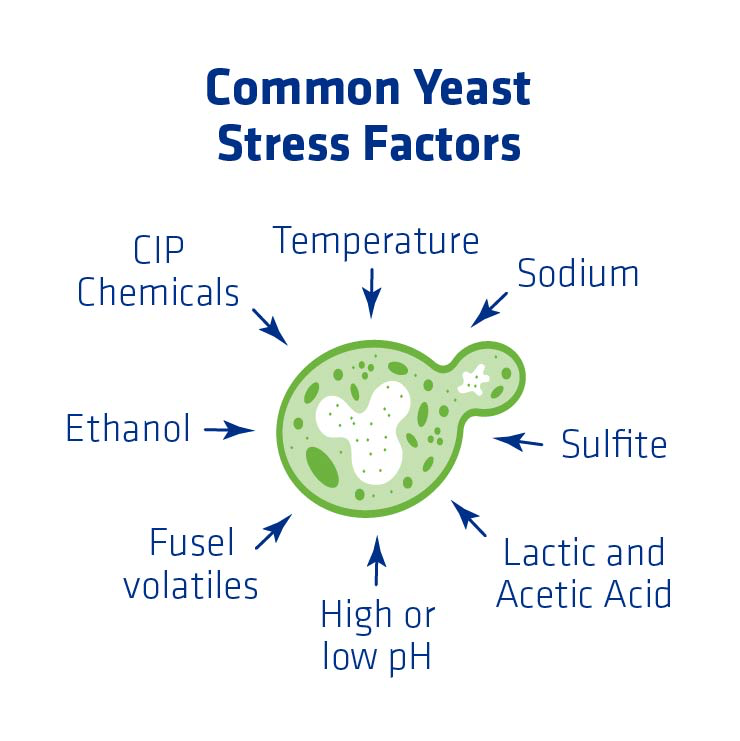
Yeast Stress Factors
As soon as yeast is introduced into the process, it is exposed to a host of stress factors at various times and in combination with one another. There are too many shown in the figure below than can be explained in this article, but the most common stress factors that will be explained in more detail are:
- Temperature
- Lactic and acetic acids
Temperature Impact on Yeast
It is critical to monitor and maintain appropriate temperatures in fermentation. When yeast are actively growing, they are more sensitive to temperature stress. As fermentation progresses and ethanol concentration rises, the optimal temperature for fermentation decreases as stress from ethanol and other harmful compounds increase.
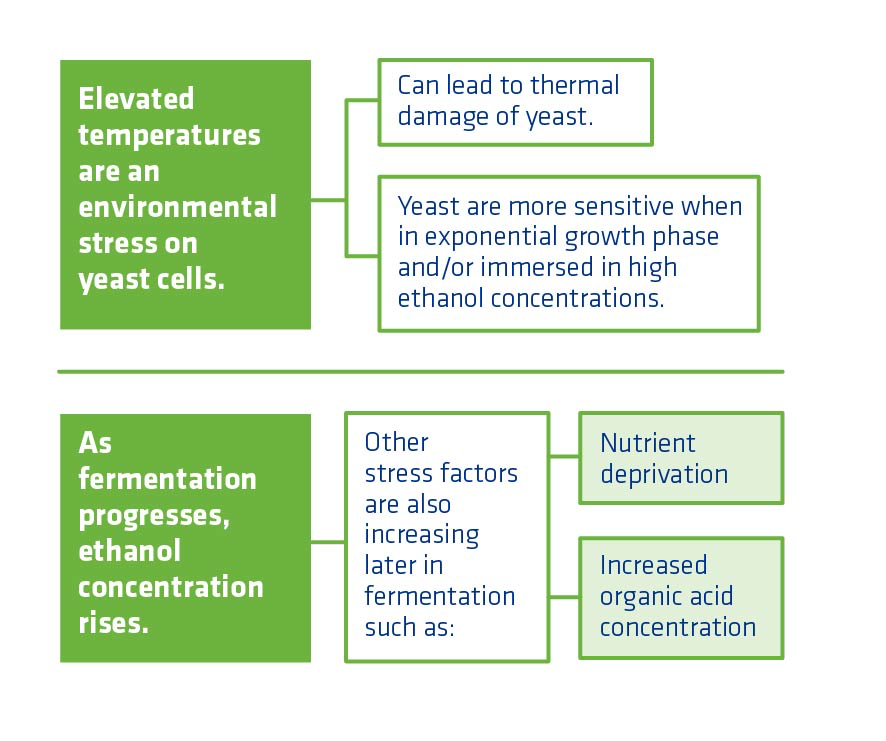
A yeast cell membrane is soft but ordered, with channels for substances coming in and ethanol going out. If the temperature increases, the membrane becomes more fluid, widening the channels. This can allow undesirable substances such as fusel alcohol and organic acids to enter the cell. It also allows substances that should be kept inside the cell to leave. In short, the yeast can no longer regulate its cell membrane. Thermal damage can also affect the proteins in the cell wall, causing more problems with yeast health.
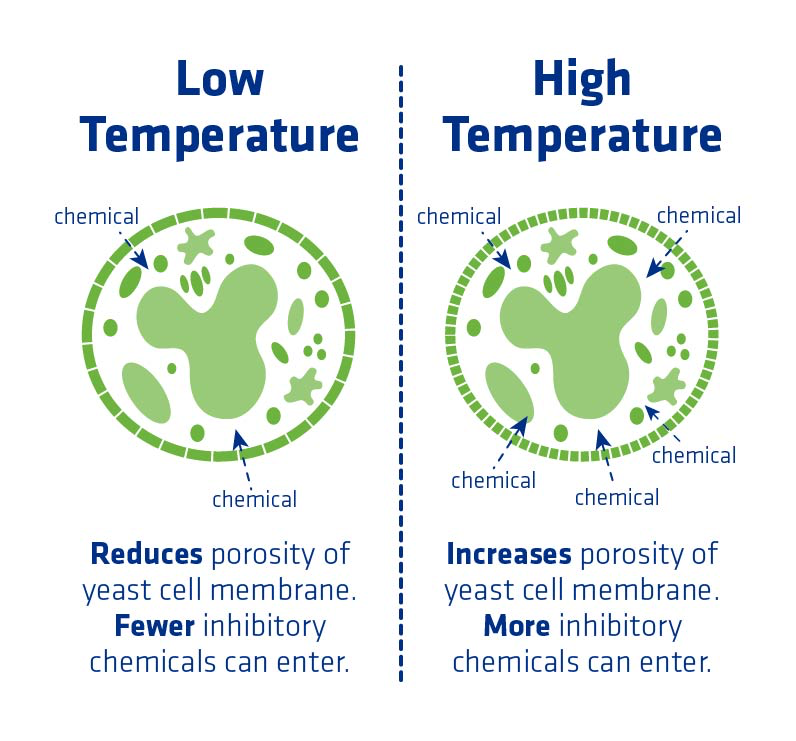
As a general rule, fermentation temperature should be kept lower than 95°F. The optimum temperature for fermentation is far lower, but is impractical for fuel ethanol applications.
Lactic and Acetic Acid Impact on Yeast
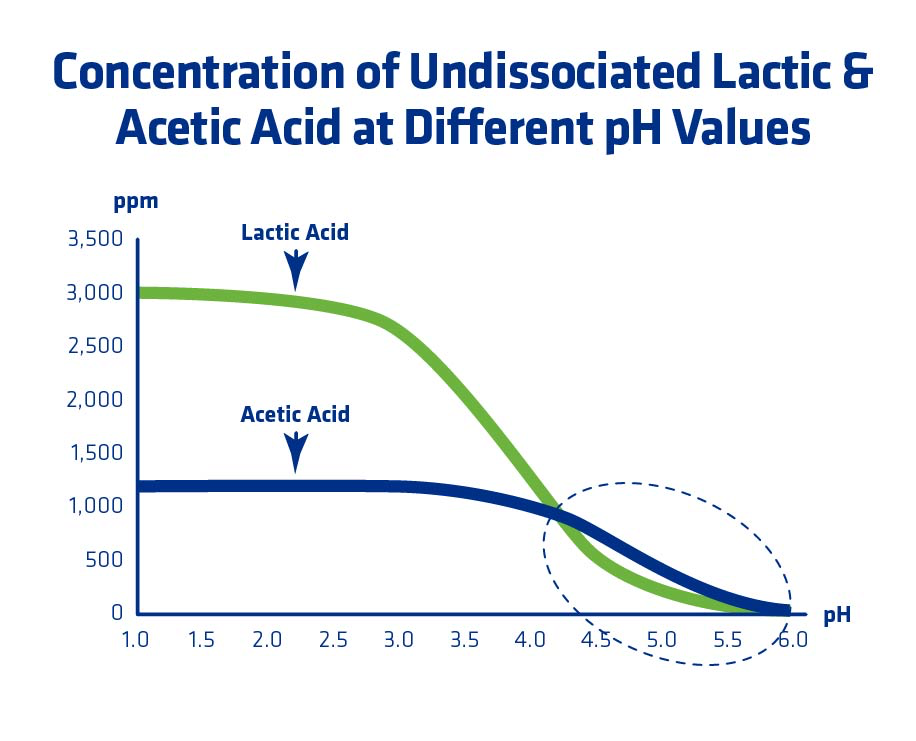
The impact of lactic and acetic acids on yeast is dependent upon pH. More specifically, the pH as it relates to the acid dissociation constant (pKa). At a pH equal to the acid dissociation constant of the acid, half of the acid is dissociated and the other half is undissociated. As the pH drops below the pKa, the more toxic it is for yeast because more acid can enter the cell. Once inside the cell, the acids lower intercellular pH. In order to regulate pH, the yeast has to spend energy (ATP) to pump the acids out, which can take energy away from ethanol production and other essential yeast cell functions (Figure 9).
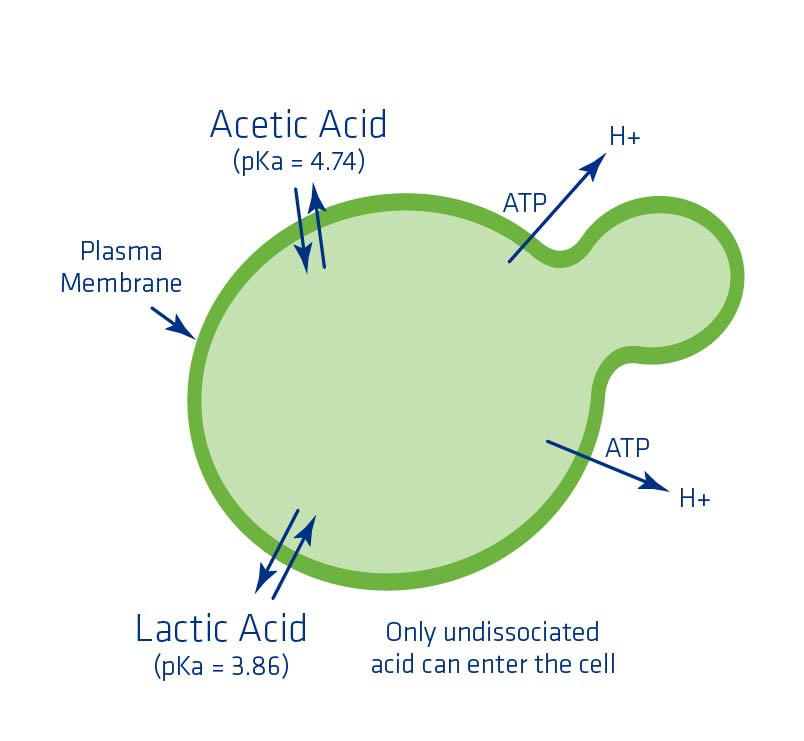
If a plant is experiencing elevated levels of lactic and acetic acids, one way to help relieve some of the acid stress would be to increase the pH slightly to help slow down the amount of acid that can enter the cell.
Phibro EPG has a calculator that uses pKa, current plant pH conditions, and lactic/acetic acid HPLC concentrations to determine the amount of acid that is undissociated and can therefore enter the yeast cell (causing additional stress). Figure 10 illustrates an example where lactic acid concentration is 0.3% w/v (3,000 ppm) and acetic acid concentration is 0.12% w/v (1,200 ppm) but at the circled pH range, undissociated levels of acetic acid are higher than lactic acid. For more information on the calculator referenced, contact your Phibro account manager.
In summary, yeast health can be achieved by providing the right nutrition at the right time and reducing yeast stress factors whenever possible. A healthy yeast population reduces the chance for bacterial infections and increases the potential for higher yield.